Eli Lilly: Addressing Provide Challenges Ought to Speed up Development (NYSE:LLY)

jetcityimage
Shares of Eli Lilly (NYSE:LLY) reached new all-time highs this week, pushed by the continued share worth and elementary momentum, and by what some would say is the weight problems market hype. The previous is true if we simply look on the numbers, however there may be additionally some reality to the latter. This may be seen by the formation of a GLP-1 ETF of which Eli Lilly and fundamental competitor Novo Nordisk (NVO) are a giant half – the Roundhill Glp-1 & Weight Loss ETF (OZEM).
The title and ticker image are short-sighted as a result of the weight problems market is already anticipated to go far past the GLP-1 mechanism and the OZEM ticker is definitely primarily based on Ozempic, which is the sort 2 diabetes model title of Novo Nordisk’s semaglutide and the precise title of the load loss drug Wegovy. Nonetheless, there may be actual substance behind the hype and each Eli Lilly and Novo Nordisk would ship much more prime and backside line progress if each of their weight problems merchandise weren’t provide constrained.
Eli Lilly’s valuation is a bit uncomfortable at present ranges, nevertheless it is without doubt one of the fastest-growing and best-positioned large pharma corporations by way of future progress prospects. There may be additionally the potential for progress acceleration and additional upside revisions as soon as the corporate secures sufficient drug provide.
Provide-constrained progress of tirzepatide (Mounjaro/Zepbound)
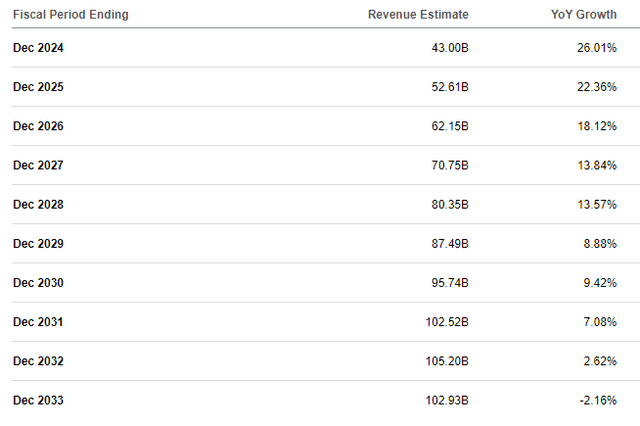
When Eli Lilly reported first quarter outcomes, it barely missed income estimates, however that didn’t cease the corporate from elevating the full-year income steerage vary by $2 billion on the mid-point to $42.4-43.6 billion. The brand new mid-point of the vary represents 26% Y/Y progress and 35% Y/Y progress if the numbers are adjusted for the current divestitures.
The non-GAAP EPS steerage was additionally elevated from $12.20-12.70 to $13.50-14.00, pushed by the elevated income progress steerage vary.
The elevated income steerage was the results of administration getting extra comfy concerning the provide outlook for tirzepatide (Zepbound/Mounjaro), however the progress of the incretin enterprise (consisting of Zepbound, Mounjaro and Trulicity) remains to be closely provide constrained. Eli Lilly continues to take a position aggressively to increase the manufacturing of tirzepatide throughout the globe and this consists of an extra $5.3 billion introduced this week to extend the capability of its facility in Lebanon, Indiana. The timelines present how ramping manufacturing isn’t straightforward and takes time – floor was damaged in 2023, manufacturing will start towards the top of 2026 and can scale up by way of 2028.
On the Q1 earnings name, administration stated that provide constraints will ease within the second half of the 12 months and that, relying on the demand and the corporate’s means to get its further manufacturing websites on-line, it might final by way of 2025 as properly.
Despite the fact that income progress estimates have risen significantly in the previous couple of quarters, I consider the market remains to be underestimating the expansion potential in 2025. This regardless of expectations of continued however much less pronounced provide constraints, additionally for the second half of the last decade as provide points needs to be addressed by then. As such, I see potential for progress acceleration in 2025 and 2026 versus an estimated progress slowdown from 26% this 12 months to 22.4% in 2025 and 18.1% in 2026.
Eli Lilly’s place within the weight problems market and progress prospects
Trying on the progress of the incretin enterprise and the evolution of the market, Eli Lilly isn’t solely maintaining, but in addition barely catching as much as Novo Nordisk. The essential caveat right here is the provision constraints for each corporations. These market share dynamics in current quarters are solely being pushed by the supply of tirzepatide and semaglutide and this may stay the case for the foreseeable future.
Eli Lilly earnings experiences, Novo Nordisk earnings experiences, creator’s calculations for Novo Nordisk incretin drug gross sales primarily based on USD/DKK change charges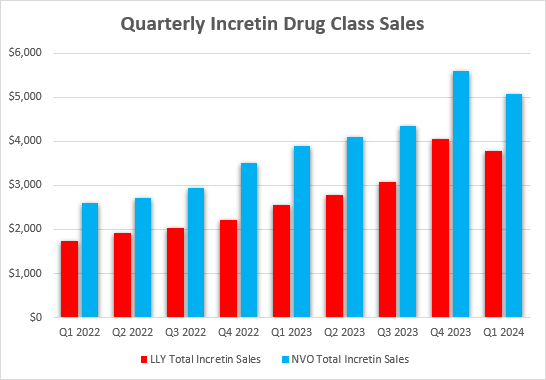
Because the graph exhibits, Novo Nordisk remains to be the weight problems and the general incretin class chief. Novo Nordisk additionally has a bonus over Eli Lilly by way of product labels as Wegovy’s label now consists of the indication of decreasing dangers of main cardiovascular hostile occasions (‘MACE”) which allows Medicare protection within the US, and Eli Lilly’s cardiovascular outcomes trial of tirzepatide is simply anticipated to finish in 2027.
Nonetheless, Eli Lilly’s benefits over Novo Nordisk embrace higher topline efficacy of tirzepatide (primarily based on cross-trial comparisons) and that is one thing the corporate intends to show in a head-to-head trial towards semaglutide with topline outcomes anticipated this 12 months.
Eli Lilly additionally reported constructive section 3 outcomes of tirzepatide in obstructive sleep apnea this 12 months, and the outcomes from a section 3 trial of tirzepatide in coronary heart failure with preserved ejection fraction (‘HFpEF’) and from the talked about head-to-head trial of tirzepatide towards semaglutide are additionally anticipated this 12 months. Constructive outcomes will probably be adopted by regulatory submissions to increase the label of tirzepatide to incorporate obstructive sleep apnea and HFpEF. These two indications are usually not solely essential from a market growth perspective, but in addition as a result of each obstructive sleep apnea and HFpEF will probably be eligible for Medicare protection.
And as talked about, each corporations are nonetheless struggling to provide sufficient of tirzepatide and semaglutide, and the present progress developments and market share dynamics are a extra a mirrored image of how a lot every firm can produce moderately than their aggressive positioning. I’d anticipate this to remain the case for no less than the subsequent a number of quarters, and buyers of each corporations ought to fear about their means to ramp manufacturing and the provision outlook updates the 2 corporations present moderately than the quarterly gross sales numbers themselves.
Given all of the demonstrated advantages of those medication, and the emergence of latest knowledge and extra potential indications, I anticipate progress expectations to proceed to advance within the subsequent few years.
So far as different opponents are involved, we’re unlikely to see anybody problem the 2 corporations on the industrial market since all others are properly behind. Nonetheless, we’re seeing rising challenges within the clinic from a number of opponents, and I coated these in my earlier articles on every of those corporations, however will summarize shortly right here:
- Amgen (AMGN) solely made qualitative feedback about its MariTide compound however prior knowledge point out this could possibly be one of many first longer-acting opponents to problem the 2 leaders.
- Viking Therapeutics (VKTX) reported important weight reduction at week 13 with once-weekly subcutaneously administered VK2735 and promising 4-week knowledge for the oral model of the identical drug. You may see my evaluation of the leads to the article I revealed this month.
- Roche (OTCQX:RHHBY) has joined the occasion because of its late 2023 acquisition of Carmot, and reported topline outcomes with important placebo-adjusted weight reduction, which I coated final week.
These are simply a few of the potential opponents and we must always anticipate many extra to emerge within the clinic within the following years. Nonetheless, all of those candidates have solely generated section 2 knowledge and are but to even begin registrational section 3 trials, not to mention a cardiovascular outcomes trial that take 5 years or longer to finish.
Nonetheless, Eli Lilly and Novo Nordisk themselves are usually not standing nonetheless and each have further pipeline efforts to additional solidify their management positions.
Subsequent in line for Eli Lilly is orforglipron, the corporate’s oral nonpeptide GLP-1 agonist, with section 3 readouts in 2025 from trials in sort 2 diabetes, weight problems, and a smaller cardiovascular outcomes trial in sort 2 diabetes sufferers. Orforglipron isn’t as aggressive on weight discount as tirzepatide, however could possibly be a superb choice for a lot of sufferers. Within the section 2 trial, orforglipron achieved weight reductions starting from 8.6% to 12.6% at week 26, or 6.6% to 10.6% placebo-adjusted.
And retatrutide, the corporate’s once-weekly subcutaneous “triple G” agonist (GLP1/GIP/glucagon) is shut behind with a lot of the section 3 readouts anticipated in 2026 and 2027. Retatrutide has an much more highly effective weight reduction impact than tirzepatide with as much as 24% weight reduction at week 48.
Past the incretin class, Eli Lilly is engaged on bettering the physique composition by rising fats mass loss and retaining lean physique mass. Final summer time, it acquired Verzenio that introduced bimagrumab. I wrote about this in my February article on Regeneron (REGN):
Bimagrumab is a monoclonal antibody that binds activin sort II A and B receptors to dam activin and myostatin signaling, and it believes that combining with incretins similar to Zepbound or retatrutide might protect muscle mass and will result in higher outcomes for folks with weight problems and with associated problems. Importantly, the addition of a drug like bimagrumab might tackle the massive disadvantage of incretins – important lack of muscle mass alongside fats loss.
Bimagrumab generated spectacular knowledge in a small section 2 trial – it led to important loss in fats mass (7.5kg versus 0.5kg fats mass loss for placebo), a acquire in muscle mass (1.7kg acquire versus 0.4kg muscle mass loss for placebo) and it additionally demonstrated metabolic enhancements in sort 2 diabetes sufferers who have been obese or overweight.
That is an rising and really attention-grabbing space within the weight problems market that I coated in better element within the above-linked article on Regeneron, and Eli Lilly has been proactive in getting its palms on bimagrumab and advancing these efforts. The subsequent step is to report the section 2 outcomes of bimagrumab together with semaglutide later this 12 months and we must always see whether or not the mixture strategy results in higher physique composition with elevated fats loss and minimal or no lean mass loss. And sure, Eli Lilly is doing a mix examine with Novo Nordisk’s semaglutide, however that is the trial Eli Lilly inherited when it acquired Verzenio and determined to finish the examine to hurry up bimagrumab’s improvement.
There are additionally different non-incretin approaches to weight reduction and a kind of is a long-acting amylin candidate eloralintide, however this one is in earlier levels of improvement and the section 2 trial was solely initiated this 12 months.
Total, I see Eli Lilly as very properly positioned to both sustain with Novo Nordisk and to doubtlessly even to leap forward in some areas of the more and more aggressive weight problems and adjoining markets.
Eli Lilly has a sturdy pipeline past weight problems and wholesome steadiness sheet do increase the pipeline and product portfolio by way of M&A
Whereas the market is basically targeted on Eli Lilly’s weight problems product portfolio and pipeline (appropriately), Eli Lilly has a sturdy pipeline in different areas. A few of the near-term progress alternatives are lebrikizumab in atopic dermatitis and donanemab in Alzheimer’s illness, offered they’re authorized by the FDA, and a few are longer-term progress drivers.
I just like the long-term potential of RNAi candidates the corporate in-licensed from Dicerna Prescribed drugs, which was, curiously sufficient, acquired by Novo Nordisk and now Novo Nordisk will get a few of the upside from the success of those candidates by way of milestone funds and royalties on web gross sales. Specifically, I just like the long-term prospects of lepodisiran, an RNAi candidate that lowers LPa which is related to elevated danger of heart problems. This can be a actually long-term driver for the reason that section 3 outcomes are usually not anticipated earlier than 2029, however we must always get a greater concept whether or not decreasing LPa works subsequent 12 months when Novartis (NVS) experiences the section 3 outcomes of its LPa-lowering candidate pelacarsen and these outcomes ought to have direct read-through to lepodisiran.
And an essential long-term driver also needs to be M&A and Eli Lilly ought to use the revenue windfall from the quickly rising weight problems portfolio to increase its pipeline to additional strengthen its aggressive place within the weight problems market and to diversify its pipeline and scale back the long-term danger of overly counting on the weight problems portfolio.
Dangers
The primary dangers for Eli Lilly are:
- The corporate not having the ability to safe sufficient provide of tirzepatide and doubtlessly different weight reduction compounds within the following quarters and years. I don’t anticipate this being a long-term downside given the investments the corporate is making, however there could possibly be hiccups alongside the best way that trigger further drug shortages or result in prime and bottom-line misses in upcoming quarterly experiences.
- Competitors is without doubt one of the key long-term dangers because the weight problems market is now most likely probably the most aggressive in the entire trade. Almost each large pharma firm will need a piece of the market, and so will many rising smaller corporations with new and revolutionary approaches. These are, nevertheless, longer-term dangers as there are unlikely to be any industrial market challenges apart from from Novo Nordisk within the subsequent few years.
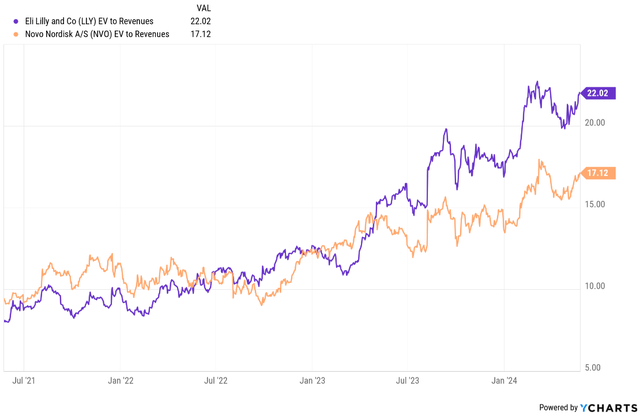
- Valuation is a big danger right here as Eli Lilly is buying and selling at elevated worth to gross sales and worth to earnings multiples, even when in comparison with Novo Nordisk. A variety of progress is priced in at present ranges and the corporate wants not solely to ship consistent with present progress expectations, it wants the earnings and income estimates to maintain trending greater to proceed to ship shareholder worth.
- There seems to be no steadiness sheet danger as of as we speak. Eli Lilly ended Q1 with $5.6 billion in money and investments and $26.2 billion in complete debt, and it ought to generate double-digit billion money flows this 12 months and much more within the following years. Longer-term, there may be the danger of the corporate making large M&A bets and stretching the steadiness sheet on offers that fail to provide the fascinating long-term upside.
Conclusion
Eli Lilly’s valuation calls for lots of progress within the following years, however primarily based on the present outlook for the weight problems enterprise and the expansion being constrained by tirzepatide’s provide constraints, I’d nonetheless anticipate the inventory to carry out properly over the subsequent few years. The corporate additionally has a wholesome medical pipeline with many further pictures on purpose that would considerably increase the income base by way of the remainder of this decade and it has the steadiness sheet to additional strengthen its product portfolio and pipeline within the following years.
Alternatively, I’m additionally conscious of the dangers and the elevated valuation and don’t intend to overstay my welcome if Eli Lilly begins falling behind on each elementary and worth fronts.
Editor’s Observe: This text discusses a number of securities that don’t commerce on a significant U.S. change. Please pay attention to the dangers related to these shares.